Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Software Requirements
| Android | iOS | Windows | MacOS | |
| with best with | Chrome | Chrome | Chrome | Chrome |
| support full-screen? | Yes. Chrome/Opera No. Firefox/ Samsung Internet | Not yet | Yes | Yes |
| cannot work on | some mobile browser that don't understand JavaScript such as..... | cannot work on Internet Explorer 9 and below |
Credits



 Written by Loo Kang Wee; Felix J. Garcia Clemente; Francisco Esquembre; Designed by David Loh
Written by Loo Kang Wee; Felix J. Garcia Clemente; Francisco Esquembre; Designed by David Loh
end faq
https://play.google.com/store/apps/details?id=com.ionicframework.covalentbonding this version has the valency question
Sample Learning Goals
- A Level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5
- O level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5
For Teachers
From Google slides (From David) to interactive https://docs.google.com/presentation/d/1fwutLc-jPc1fUyrxJsps3Fhg6_J9y8AEA4pHJ68gdBw/edit?ts=5dd2086a#slide=id.g5292a6c619_0_96
If you are using this simulation in Singapore Schools, please contact This email address is being protected from spambots. You need JavaScript enabled to view it. to get a digital google survey form to collect anonymous data on the effectiveness of lesson and future imporvement to the simulation!. Thanks in advance.
Our own survey suggests strong evidence on effective student learning, appreciative students, good simulation design. Do you agree? let me know in the comments below! https://weelookang.blogspot.com/2020/02/o-level-chemical-bonding-dot-and-cross.html
Chemical Bonding Dot and Cross Diagrams
Polyatomic ions dot and cross diagram
|
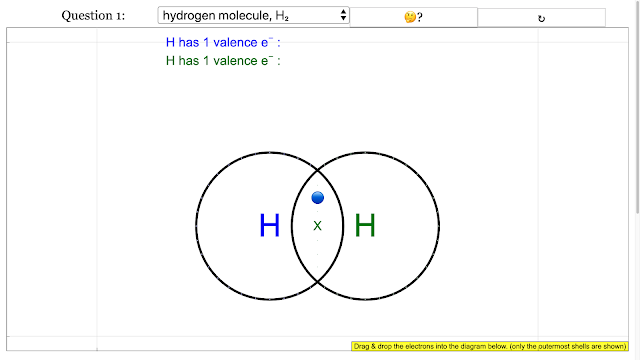
Chemical Bonding Dot and Cross Diagrams for Hydrogen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen molecule answer is add up to 2 on each H atom electron outermost with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Chlorine molecule |
|
Chemical Bonding Dot and Cross Diagrams for Chlorine molecule answer is add up to 8 on each atom's' electron outermost shell with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Oxygen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Oxygen molecule answer is is add up to 8 on each atom's' electron outermost shell with shared electrons = 4 |
|
Chemical Bonding Dot and Cross Diagrams for Nitrogen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Nitrogen molecule answer is is add up to 8 on each atom's' electron outermost shell with shared electrons = 6 |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Chloride molecule |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Chloride molecule answer is is add up to 8 on Cl atom and 2 for H atom and electron outermost shell with shared electrons = 2 |
|
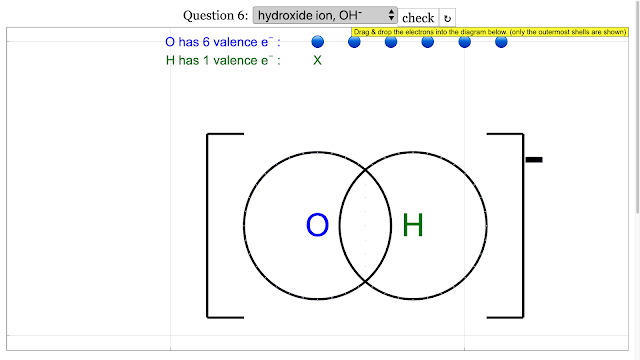
Chemical Bonding Dot and Cross Diagrams for Hydroxide ion |
|
Chemical Bonding Dot and Cross Diagrams for Hydroxide ion answer is is add up to 8 on O atom and 2 for H atom and electron outermost shell with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Cyanide ion |
|
Chemical Bonding Dot and Cross Diagrams for Cyanide ion answer is is add up to 8 on each atoms and electron outermost shell with shared electrons = 6 |
|
Chemical Bonding Dot and Cross Diagrams for Water molecule |
|
Chemical Bonding Dot and Cross Diagrams for Water molecule answer is is add up to 8 on O atom and 2 on H atoms and electron outermost shell with shared electrons = 2,2 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Carbon Dioxide molecule |
|
Chemical Bonding Dot and Cross Diagrams for Carbon Dioxide molecule answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 4,4 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 2,4 respectively with the foreign electron on the O atom with the shared electrons=2 |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 2,4 respectively with the foreign electron on the O atom with the shared electrons=2 |
|
Chemical Bonding Dot and Cross Diagrams for Ammonia molecule |
|
Chemical Bonding Dot and Cross Diagrams for Ammonia molecule answer is is add up to 8 on N atom and 2 on H atom and the electron outermost shell with shared electrons = 2,2,2 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Methane molecule |
|
Chemical Bonding Dot and Cross Diagrams for Methane molecule answer is is add up to 8 on C atom and 2 on H atom and the electron outermost shell with shared electrons = 2,2,2, respectively |
|
Chemical Bonding Dot and Cross Diagrams for Carbonate ion |
|
Chemical Bonding Dot and Cross Diagrams for Carbonate ion answer is is add up to 8 on each atom and the electron outermost shell with shared electrons = 4,2,2, respectively. The two O atoms with 2 shared electrons has a foreign electron in it's personal shell. |
 |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Peroxide |
Student Survey
survey 1 , 2 data from MGS 2020 total 1 + 2 classes estimated number of students (44 , 46) responses.
I have added some answers to the questions asked too.
 |
| typical classroom setting where the anonymous (photo blurred) teacher skillfully explains the learning tasks using https://iwant2study.org/ospsg/index.php/922 |
 |
| typical classroom setting where the anonymous students are each working on their ChromeBook to learn by doing chemistry. this is far better than students working on Google Slides and trying to fit in the electrons on non simulation medium, only getting feedback on the accuracy after the teacher manually marked each students' assigned google slide prepared by the teacher. https://iwant2study.org/ospsg/index.php/922 |
What do you like about the lesson? (For example, was the lesson successful in helping you to learn the concepts? In what way was the simulation helpful? Did it arouse your interest? Please give more details.)
- It was successful in helping me learn the basic concepts but it did not give me a very clear understanding of the concept. I was still left with questions and doubts. It was a unique and new way of learning in the classroom. noted, Video Tutorial will be ready after march holidays.
- Something new. No need to check the periodic table. There are other compounds not tested available. The emoji is cute.
- It was helpful to visualise the concepts.
- the lesson was successful in helping me to learn the concepts. the simulation was helpful. It arouse my interest.
- it is interesting and can help me learn independently.
- The lesson was more hands on than just the teacher explaining to us how to draw dot and cross diagrams
- This is a more interesting style of learning
- The simulation made the lesson more interesting as it was interactive
- the simulation was easy to use and easy to understand
- The simulation helps me to understand better about chemical bonding dot & cross diagram. I also can engage with the lesson better because we get to use technology during lessons and there are colours.
- yes,it helps me a lot ,I was confused about the definition yesterday night ,but through this lesson, I think I know better about the definition and the process .
- it helped me to understand better
- the simulation made me more interested in learning about chemical bonding.
- I like the fact that we got to learn through using our chromebook and got to try it out ourselves.
- it allowed me to understand more about the formation of covalent bonds
- I was fun and was an easier and more interesting way to learn about covalent bonding instead of literally drawing the atom over and over again.
- It keeps my interest because it is easy to use
- it was interesting i guess
- It was interesting and helped me to be more confident in dot and cross diagrams.
- that we get to attempt it ourselves first
- It is easy to use and informative
- it was easy to understand with answers and hints provided
- The simulation was cute and captured my attention so I enjoyed doing it.
- The simulation allowed me to discover where the electrons should go and somehow, why it was like that.
- it's easier to use
- it was easy and fun to use
- The lesson was successful in helping me learn the concepts. It gave me more practice in covalent bonding.
- the simulation gave me a concept of what covalent bonding is about
- The simulation is much easier than drawing the atoms out.
- I like that the lesson allowed me to learn independently and the diagrams were simple and easy to read.
- The lesson helped me have a clear visual on covalent bonding. Helped it to be clearer to me.
- The answers are not given on the first wrong attempt, we can continue to try to get to the correct answer.
- The simulation made the locations of the electrons clearer
- I like that it gives the number of electrons that the user has to use and that the user does not need to refer to the periodic table to do this simulation, increasing interest and ease in doing this while learning something.
- It was a refreshing change. Instead of drawing the diagrams on paper, I could move the electrons online.
- it is very entertaining and educative
- the visuals helped me!
- it was interesting
- The colour coding makes the visuals much more easier to see and understand the concepts.
- The lesson was very fun and engaging. The simulation allowed me to better understand the concepts.
- It was successful in helping me learn the concepts as it provided a solution and explanation when i didn't know how to do the question.There were also many question which allowed me to try out different questions or different levels of difficulty,allowing me to practice more.
- it was fun
- The hints especially helped me to understand the concept
- I can understand the structures more clearly. was quite fun to drag and drop instead of drawing for once. It's also much more convenient as i can check my work immediately instead of waiting for the teacher to come around
- The simulation was easy to use and was a good reference for doing the worksheet as well !
- the simulation was helpful in giving model answers for me to understand covalent bonding better
- The simulation helped me determine the number of valence electrons so it was easier to draw the dot and cross diagram. The simulation was very fun and easy to use and it was a drag and drop application
- was very successful
- the simulation helped me better understand the topic better and it was engaging
- I liked that it was more engaging and gave us room to think for ourselves while having some guidance from the simulation. The simulation made it easier to trial and error so that I could figure out the bonding by myself.
- it is interactive and clear:)
- It helped me understand the concept of bonding better. It was very interesting and helpful. I would like to do it again in class sometime.
- the lesson was easy to follow since the simulation was easy to use and we could just reset instead of redrawing
- It was a lot easier to correct small errors using the simulation that it is with pen and paper.
- It was pretty interactive and it helped us to apply important concepts when drawing dot-cross diagrams.
- - easy to use
- - I had more confidence in drawing dot and cross diagrams
- - wide range of ions to choose from
- - "hints" feature
- It was easy to use and a suitable amount of help was given (number of valence electrons per element were given)
- i liked how it was not too complicated to do the dot-and-cross diagrams and the website was easy to use.
- helpful - it prompted us and gave hints on how to draw the diagram
- yes i am interested
- it was good to check whether my diagrams were right
- Yes it was very helpful. I learnt how to draw polyatomic ions very quickly and I find this kind of simulation useful as I can self-learn without relying so much on the teacher. :)
- It gives a better visualisation of the dot and cross diagram and helps me to be able to draw the diagram easily.
- It made the process of thinking how to draw it much easier
- i was able to learn the concept faster with the use of the website
- It helped me visualise the covalent bonding between the molecules and made me curious to find out why the molecules bonded in a particular way
- I liked using the simulation to trial and error with the diagram and to fill in the worksheet.
- the platform made learning the dot cross diagram more interesting
- it helped me figure out the answer in my own.
- the lesson helped to make my understanding of covalent bonding clearer
- I liked the idea of figuring it out yourself and then having a check answer button this app is great!
- It made visualising the covalent bonds and the orientation of electrons easier.
- ー
- it is fun to drag the electrons around
- it gives me many chances for trial and error without having to continuously erase my mistakes
- I like that we get to explore the concept ourselves and learn by trial and error.
- The simulation was easy to use and allowed me to understand how to draw dot and cross diagrams as it told me what was wrong when I made a mistake
- I learnt how foreign electrons worked, and it was interesting to see try doing the different questions. It was also easier as the simulation already had the electrons shells put out for you.
- It helped me to visualise the concept better
- I liked the lesson because it was more engaging and hands-on, and it also helped me to learn the concepts. It is also better than having to draw out a dot and cross diagram myself.
- It helped me to see the chemical bonds visually which was very helpful
- It allowed me to easily move each electron around before writing it on my paper thus it was easier and neater. Furthermore, because of the difference colours it is easier to see the electrons
- The simulation made it easier for me to visualise the atoms, aiding me in figuring out the configuration of different compounds.
- It was successful in conveying the concepts of chemical bonding. It allowed me to explore the concept better.
- It allowed me to understand the concept better as it gave an explanation when providing the answer which was more comprehensive then just searching for the answer online.
- it was interactive and helped me to understand the lesson better
- I think it helps me understand where I went wrong and teaches me how to correct my mistakes before I write it down and have to go through the trouble of changing it over again and again.
- it was fun and easy to use!
- kept me engaged and aroused my interest
- it was successful because it was fun and interesting
In what way can the lesson or the simulation be improved? (For example, what features in the simulation would you like to change or add?)
- NIL
- Dragging and clicking of the electrons became tiring after a while, and it was very repetitive and could be more engaging. noted, We already designed a book icon that put all the electrons in appropriate positions, maybe need to tell students again?
- An option to include non-valence shells and a box of different colours of ions(we choose how many to take) but include the periodic table at the side.More effects and colours so it will be more attractive. If you can, thank you! noted, while the idea has merits, it may be too difficult when there is a unknown number electrons to position on the atomic shell, so it is unlikely i will implement that idea.
- It is a bit hard to drag the dots and crosses to their respective molecule. noted i have increase the sensitivity to 50px, meaning the dragging area is now 50x50px on the electrons O and X.
- the simulation can have more.
- -nil-
- We can only place the dots and crosses at certain places of the ring
- add a function that could help memorise the formulas or dot and cross diagrams better
- I think it is already very good.
- figure out how to make those who do not have touch screen laptop use more easily
- -
- it is slightly hard to drag the electrons and place them on their spots
- more graphics can be added to make the site more interesting.
- -
- Harder questions.
- Make the colours of the molecules in the simulation brighter as some were dull and
- i feel that the moving of dots was quite tiring and tedious
- The lesson can have more of these interactive games to keep us engaged.
- instead of drag and drop change it to click to grab and click to drop
- the simulation should have a function that allows us to pull all the electrons to the outer circle so we do not have to do it one by one.
- I would like there to be an explanation added in if the hint is used as seeing the answer alone does not help me understand the bond.
- make it easier to drop the electrons
- There can be some pop ups where the user can see what that compound is applied in daily lives so to spark more interest in learning these compounds:))
- it could be more aesthetically pleasing
- the toggle was a bit weird
- the electrons can be larger so it's easier to drag.
- NIL
- I think the thinking emoji can be changed to something like "Check" because some people didn't really know what to press to check the answer immediately. noted, i have added text check hint and reset.
- I find it tedious to move every single subatomic particle to its spot! I'd rather have them assigned to their respective atom and then move them around from there !
- make it more appealing including the colour lilac
- Instead of dragging the ‘x’ or the ‘o’ , we can tap them to move them from one place to another.
- there could be an explanation for why some ions are bonded that way
- nil
- - labels for buttons. done
- - better interface design
- Just select directly on the diagram instead of dragging it as it is quite tedious without touch screen.
- explanations should be given when students gets the answer incorrect.
- how to derive the answer
- Easier dragging of electrons cause sometimes it lags.
- More explanation (rgd concepts) please! Thank you! (esp for the last few qns)
- make it easier to drag and drop the electrons?
- There can be an explanation for the bonding after students submit each answer.
- would it be possible to have a wider range of compounds that we can try to do?
- it would be nice if there was an undo button rather than completely erasing what you've done, also its a bit tedious having to drag things over it may be nice if you can select an electron and place it in a spot
- pop up tutorial of how to use it. I wasn't really sure about how to check my answers. video coming
- more variety of compounds
- more graphics?
- The simulation could have a one step 'backspace' key instead of a total reset so we can undo step by step and do not have to reset when we want to backtrack.
- The simulation is really good and there is no improvements that I would want to see made.
- more covalent bonds/questions as the range of selection is small right now
- label the buttons as some students were confused on how to check the answer noted
- The simulation is a bit laggy.
- I think the explanations as to why our answers were wrong can be clearer as I was a bit confused when it first came out and didn't really understand what to do.
- the simulation could be more colourful
- automatically go on to the next qn
Any other feedback or comments for your teacher or the simulation designer? (This question allows students to feedback on any other matters. For example, you could thank your teacher or say how you feel about this simple technology enhanced lesson.)
- NIL
- -
- This app is really interesting. Thank you for spending time to make this app!
- it is fun
- -nil-
- I think the teacher should explain it more clearly first because I actually don't understand it before having to explore the simulation. I feel appreciative that the simulation designer designed this thing that helped me to understand more about the topic.
- quite great
- -
- Using technology during lessons is more fun. :)
- :D
- The simulation was really cool! Thank you so much!
- Thank you Ms T for giving us time to try out this simulation and allowing us to learn on our own! :)
- The simulation is much faster and more efficient than physically drawing covalent bonds.
- Thank you for the lesson!
- nil
- This simulation is a really great way to get students to practise and they can see how to draw and where to put the electrons in the correct places!!
- it is quite hard to drag the electron , noted sensitivity set to 50 px
- thank you for the lesson
- thanks haha:))
- NIL
- Thank you Ms T for trusting us to do our own self- learning ! Despite you not actively teaching us, it was a simple but fruitful, hands-on lesson ^-^
- thank you v much heehee
- -
- -
- thank you for the website
- THANk You MS C T
- i enjoyed the lesson using the simulation very much! thank you!
- thanks
- Thank you for letting us use this simulation.
- It's really good :)
- Thank you for creating this!
- Thank you for spending so much time and effort to create this simulation make our lessons more interesting!
- This lesson was very fun! Thank you for making the simulation!!
- thank you for this simulation. it was simple but effective
- thank you for making this programme !
- Would it be possible to have the electrons of the atoms to be an option to click on before filling in the configuration,so we don't have to drag the ones given to their rightful place?
- I like this.
- N/A
- it was really fun!!!
Video
Version:
- https://weelookang.blogspot.com/2019/12/chemical-bonding-dot-and-cross-diagrams.html
- https://sites.google.com/a/mgs.sch.edu.sg/about-me-david-loh/
Other Resources
[text]

.png
)