About
Topics
- Chemical Equations
- Conservation of Mass
Description
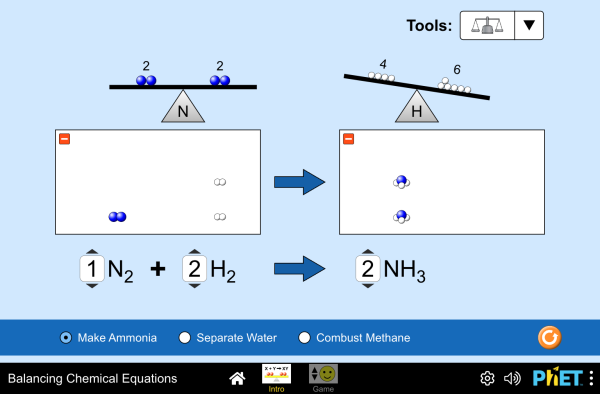
How do you know if a chemical equation is balanced? What can you change to balance an equation? Play a game to test your ideas!
Sample Learning Goals
- Balance a chemical equation.
- Recognize that the number of atoms of each element is conserved in a chemical reaction.
- Describe the difference between coefficients and subscripts in a chemical equation.
- Translate from symbolic to molecular representations of matter.
For Teachers
Video
https://www.youtube.com/watch?v=GneOr6091qU
Software Requirements
| Windows 7+ | Mac OS 10.7+ | iPad and iPad Mini with iOS | Chromebook with Chrome OS |
|---|---|---|---|
|
Internet Explorer 10+
latest versions of Chrome and Firefox
|
Safari 6.1 and up
latest versions of Chrome and Firefox
|
latest version of Safari | latest version of Chrome |
Credits
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|
|
end faq
Testimonials (0)
There are no testimonials available for viewing. Login to deploy the article and be the first to submit your review!
You have to login first to see this stats.