About
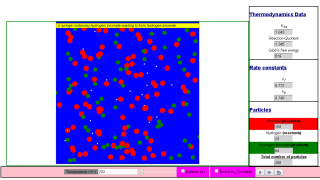
This simulation emulates the gas phase equilibria between hydrogen, bromine and hydrogen bromide molecules.
http://weelookang.blogspot.sg/2014/12/hydrogen-bromine-hydrogen-bromide.html
H2 + Br2 ⇌ 2HBr (∆H = -103 kJ mol-1)
Constants:
kB = R / NA = 1.38060445E23
R = 8.314
NA = 6.022E-23
Parameters:
Hydrogen are white particles. (mass = 2*1.00794 u)
Bromine are red particles. (mass = 2* 79.904 u)
Hydrogen bromide are green particles. (mass = 79.904 + 1.00794 u)
N = 100 molecules
H-H bond: 436 kJ mol-1
Br-Br bond: 193 kJ mol-1
H-Br bond: 366 kJ mol-1
∆H = -103 kJ mol-1
kf = Aexp(-629/8.314/T)
kb = Aexp(-732/8.314/T)
Keq = kf/kb = exp(103/8.314/T)
Fixed relations
v = Math.sqrt(3*8.314/6.022*10E23*T/mass)
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Software Requirements
| Android | iOS | Windows | MacOS | |
| with best with | Chrome | Chrome | Chrome | Chrome |
| support full-screen? | Yes. Chrome/Opera No. Firefox/ Samsung Internet | Not yet | Yes | Yes |
| cannot work on | some mobile browser that don't understand JavaScript such as..... | cannot work on Internet Explorer 9 and below |
Credits


![]() Andy Luo Kangshun; Paco; Wolfgang
Andy Luo Kangshun; Paco; Wolfgang
end faq
Testimonials (0)
There are no testimonials available for viewing. Login to deploy the article and be the first to submit your review!
You have to login first to see this stats.