Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Software Requirements
| Android | iOS | Windows | MacOS | |
| with best with | Chrome | Chrome | Chrome | Chrome |
| support full-screen? | Yes. Chrome/Opera No. Firefox/ Samsung Internet | Not yet | Yes | Yes |
| cannot work on | some mobile browser that don't understand JavaScript such as..... | cannot work on Internet Explorer 9 and below |
Credits


![]()
 This email address is being protected from spambots. You need JavaScript enabled to view it.; Anne Cox; Wolfgang Christian; Francisco Esquembre
This email address is being protected from spambots. You need JavaScript enabled to view it.; Anne Cox; Wolfgang Christian; Francisco Esquembre
end faq
Apps

https://play.google.com/store/apps/details?id=com.ionicframework.rutherfordapp857799&hl=en
Introduction
The Geiger–Marsden experiment(s) (also called the Rutherford gold foil experiment) were a landmark series of experiments by which scientists discovered that every atom contains a nucleus where its positive charge and most of its mass are concentrated. They deduced this by measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The experiments were performed between 1908 and 1913 by Hans Geiger and Ernest Marsden under the direction of Ernest Rutherfordat the Physical Laboratories of the University of Manchester.
The popular theory of atomic structure at the time of Rutherford's experiment was the "plum pudding model". This model was devised by Lord Kelvin and further developed by J. J. Thomson. Thomson was the scientist who discovered the electron, and that it was a component of every atom. Thomson believed the atom was a sphere of positive charge throughout which the electrons were distributed, a bit like plums in a Christmas pudding. The existence of protons and neutrons was unknown at this time. They knew atoms were very tiny (Rutherford assumed they were in the order of 10−8 m in radius[1]). This model was based entirely on classical (Newtonian) physics; the current accepted model uses quantum mechanics.
Thomson's model was not universally accepted even before Rutherford's experiments. Thomson himself was never able to develop a complete and stable model of his concept. A Japanese scientist named Hantaro Nagaoka rejected Thomson's model on the grounds that opposing charges cannot penetrate each other.[2] He proposed instead that electrons orbit the positive charge like the rings around Saturn.[3]
Rutherford thus rejected Thomson's model of the atom, and instead proposed a model where the atom consisted of mostly empty space, with all its positive charge concentrated in its center in a very tiny volume, surrounded by a cloud of electrons.
Video
Rutherford's Experiment: Nuclear Atom by uploaded by HerrPingui
Rutherford experiment animation by owigger
Rutherford Gold Foil Experiment - Backstage Science
Rutherford Scattering by xmphysics
Version
Other Resources
- Understanding Rutherford's gold foil experiment by Concord Consortium. Rutherford shot alpha particles at atoms to see if the positive part of atoms would interact strongly with the positve alpha particles. Explore how concentrating positive charge affects the electirc field generated by that charge and how that field affects the path of the positive alpha particles.
- JJ Thomson wrong model http://www.kcvs.ca/site/
projects/physics_files/ rutherford/thomson_model.swf - Rutherford experiment result and new model http://www.kcvs.ca/site/
projects/physics_files/ rutherford/nuclear_atom.swf - https://phet.colorado.edu/en/simulation/rutherford-scattering by PhET
Assessment
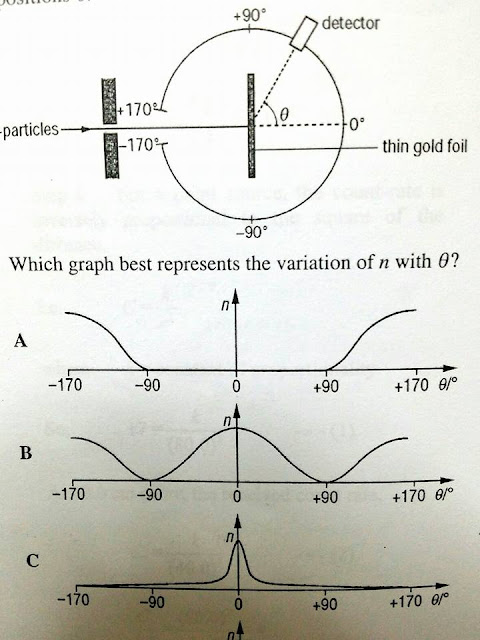 |
| assessment question |
end faq
Testimonials (0)
There are no testimonials available for viewing. Login to deploy the article and be the first to submit your review!
You have to login first to see this stats.

.png
)



