Titration Curve of Strong Acid against Weak Base
(image)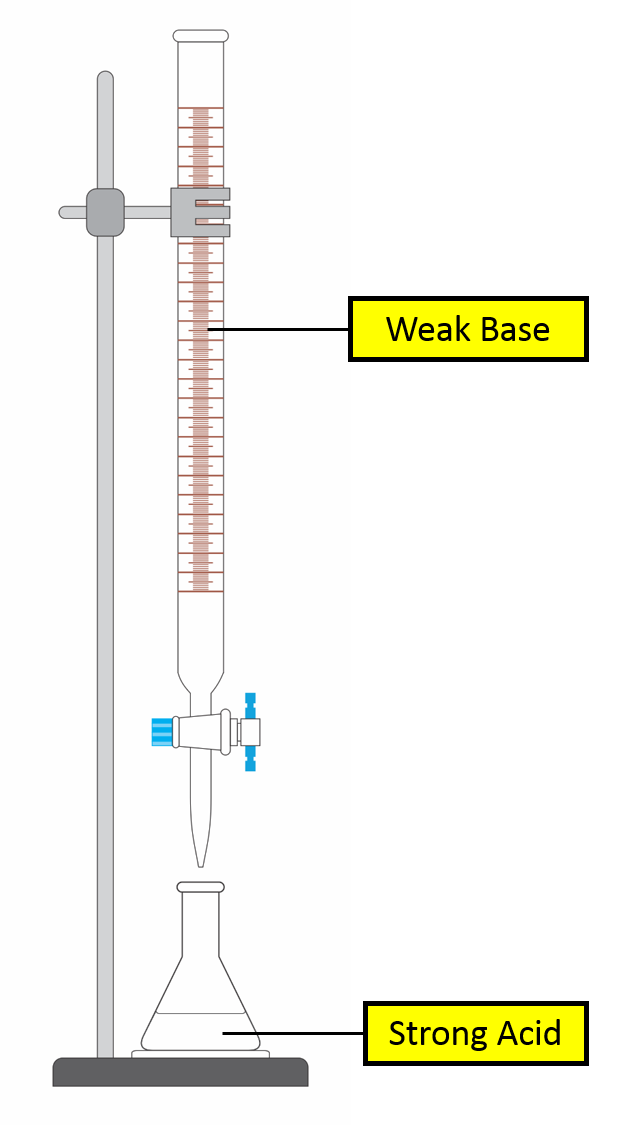

| In the burette | ||
|---|---|---|
| [base] = | mol dm-3 | |
| Kb (scientific notation) = | mol dm-3 | |
| pKb = | ||
| In the conical flask | ||
| [acid] = | mol dm-3 | |
| Strong acid is | ||
| Vol of strong acid | cm3 | |
| Choice of indicator | ||
| Graph | ||
| Vol of weak base req for complete neutralisation | cm3 | |
| Data points in increments of | cm3 | |
| Stop titration at (for auto titration only) | cm3 | |
|
|
||
Self Check
1. Select a point by clicking the graph:Burette Volume pH
2. Identify the species present in the conical flask at that point:
3. Is solution a buffer?
Quiz
| Burette Vol | pH | Total Vol |
|---|