Titration Curve of Strong Base against Weak Acid
(image)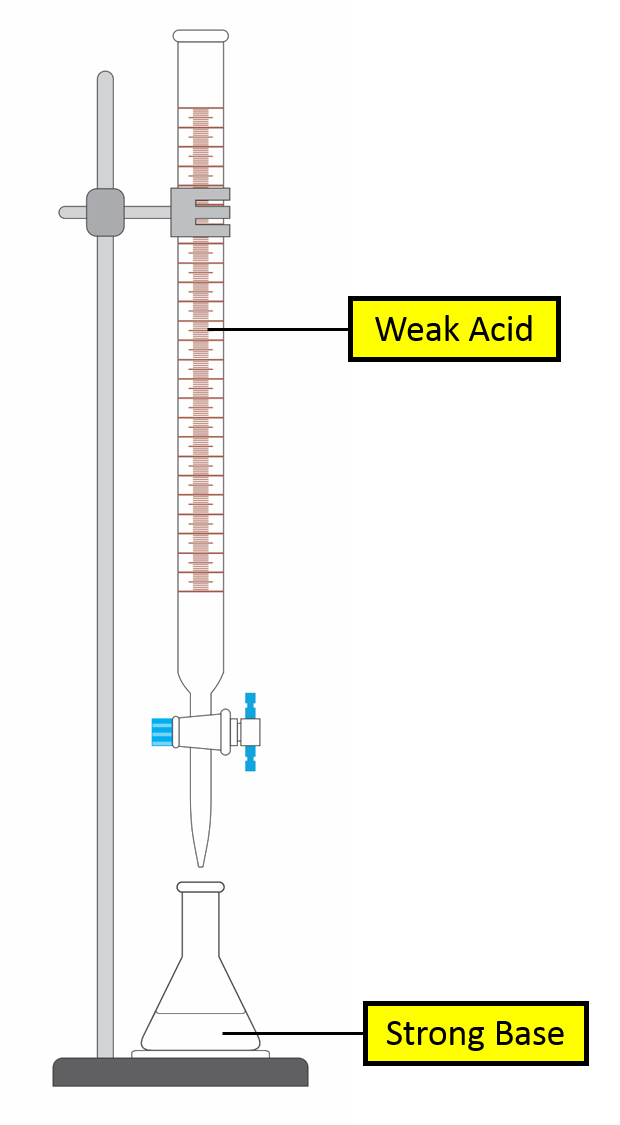
Titration Curve of Strong Base against Weak Acid
(image)
|
Self Check 1. Select a point by clicking the graph:Burette Volume pH 2. Identify the species present in the conical flask at that point: SB conj base of WA xs WA 3.Is solution a buffer? yes |
|
||||||||||||||||||||||||||||||||||||||||||||||||||