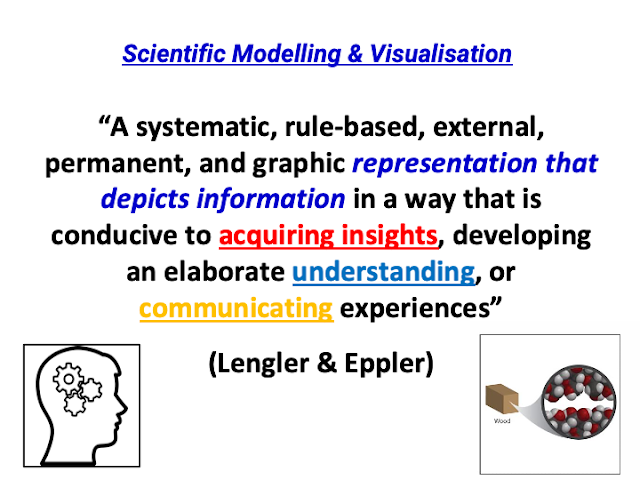
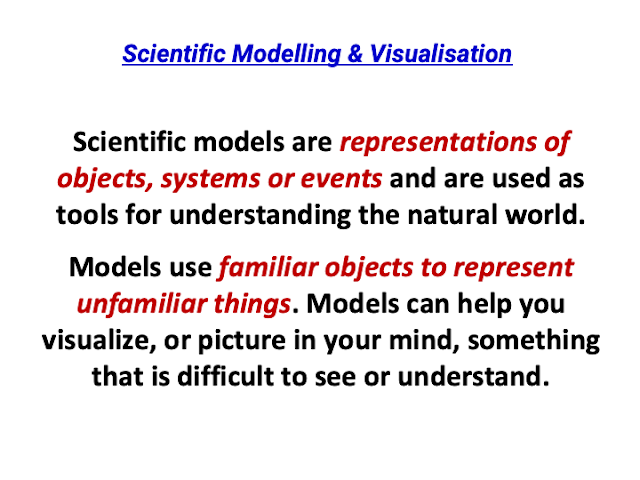
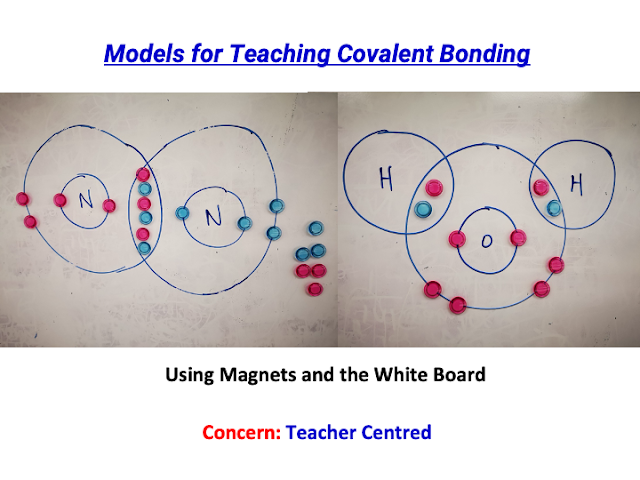
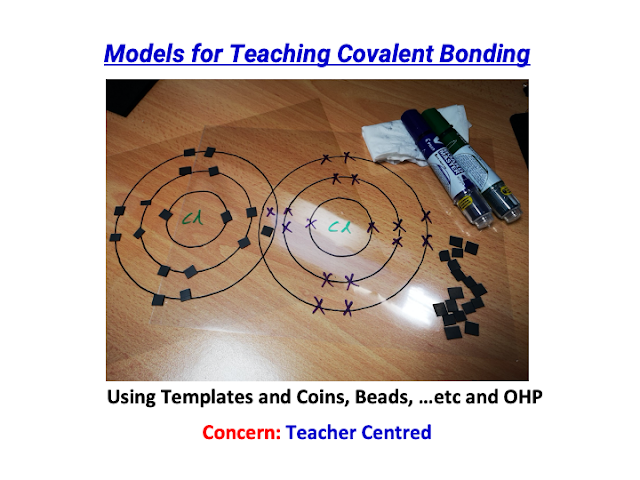
awesome sharing by David!
enjoy!
 |
| video is private, sorry! |
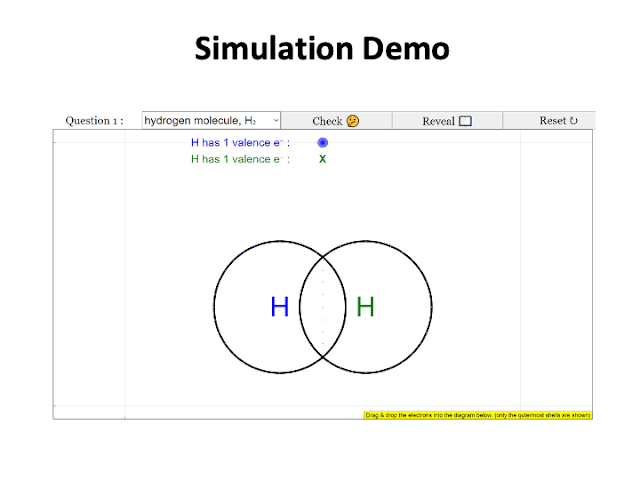 |
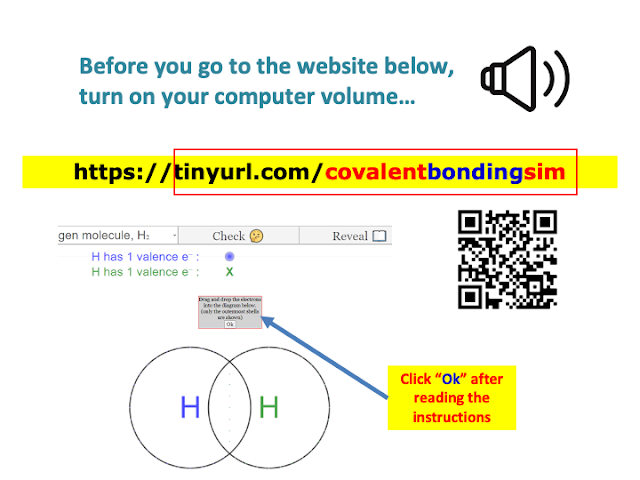
| https://iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
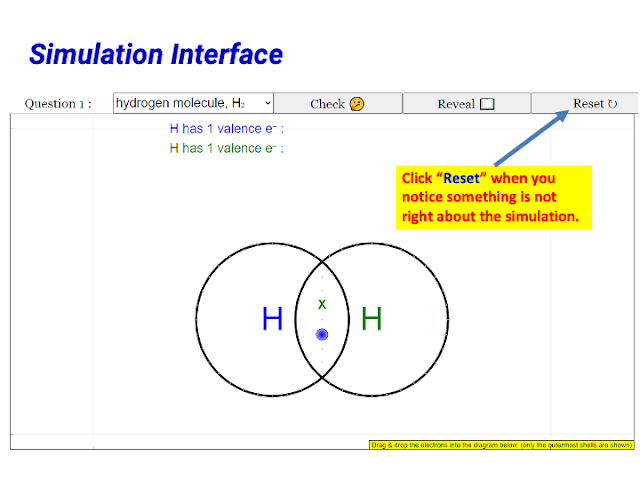 |
| https://iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
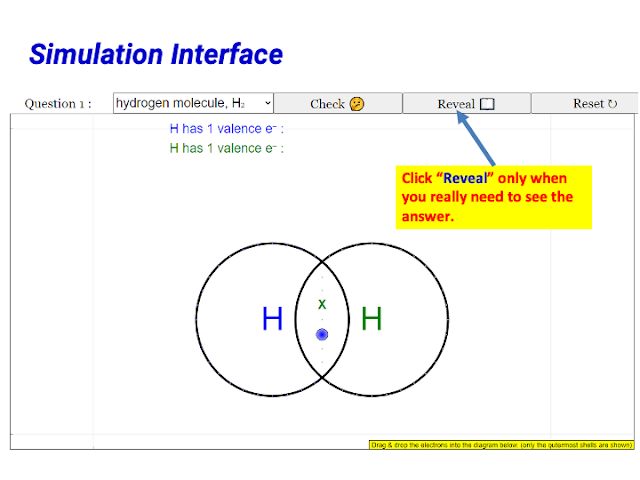 |
| https://iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
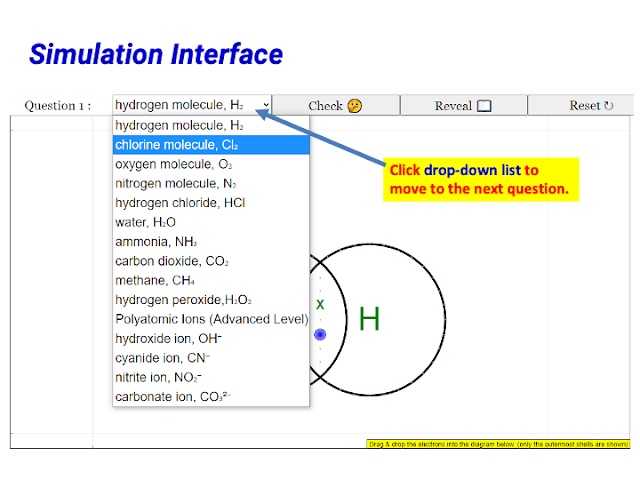 |
| https://iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| https://iwant2study.org/ospsg/index.php/922-dotandcrossdiagram8 |
 |
| results https://docs.google.com/forms/d/1qF5CuDpEol4pypxJtz9eTGdajF_pI_yGJxuoi0i1U8o/viewanalytics |
What I like about the simulation?
- Engaging for students as compared to current teaching methods. Looking forward to the ionic bonding version. I will try it in the next fortnight with both Sec 3 Express and Sec 4 Express students
- Self explanatory.. a variety of answers accepted.
- Student centered especially to higher ability students
- Instant feedback, can embed in sls
- I like that it gives students the opportunity to explore the bonding on their own and to allow students to understand the concept of the number of valence electrons that each element has.
- Easy to use. And I really like that we do a post activity test to check the students learning.
- This promotes self-directed learning.
- Engaging for students, allows students to learn independently
- Hands on and students can obtain immediate feedback for their answer .
- simple to use
- It can help students in their self-directed learning.
- Immediate feedback
- That the learning experience is different from a regular chalk-and-talk in class, students can be more self-directed.
- Self directed, good for revision as well
- It’s user friendly and the explanation given is clear too
- Easy to manipulate.
- I like that it is online. can easily accessible.
- Good for beginner students as the structure is there. They just need to figure out where the electrons go.
- very user friendly
- I think it is very easy for students to use.
- Flexibility allowed - even though electrons are paired, still accepted as good answers.
- It offers feedback to the students and let them explore.
- Concurrently hands on try by all students - can listen to the sound of their responses in the IT gadgets to judge mastery level
- User friendly and not complicated with too many functions
- Easy and simple to use. Very visual and interactive for students to learn dot and cross diagram.
- The feedback given when answer is wrong
- Easy to use
- Interactive
- It makes it easy for pure chemistry students to realise that foreign electrons are involved in polyatomic ions.
- Students can explore on their own and understand covalent bonding better
- Easy to use, clean UI
- User friendly, fuss free
- Very user friendly, creates opportunity for self exploration and can learn the concept from mistakes.
- It is good for them to see whether the student are making mistakes or not.
- Interactive and intuitive
- Instant feedback and subtle scaffolds built-in.
- Easy for students to visualise and manipulate to get the bonding right.
- User friendly, easy to embed, easily available for students and it is really extraordinary effort by David and team to work on this!
- It includes polyatomic ions which is often promotes higher order thinking. It also give immediate feedback to students' answer
- Students can learn at their own pace.
Other Feedback, Suggestions or Areas for Improvement:
- Nil
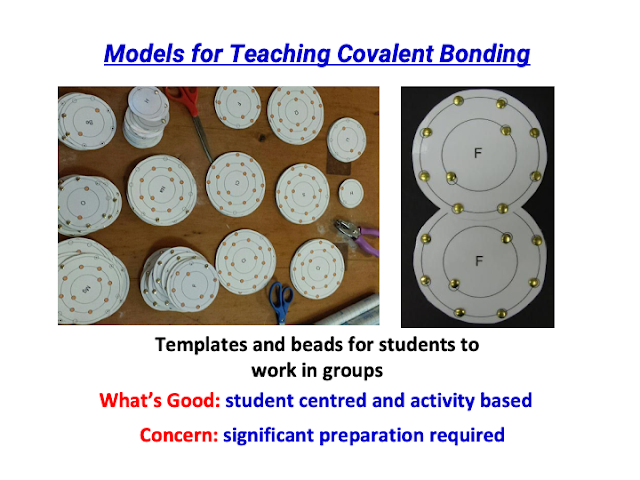
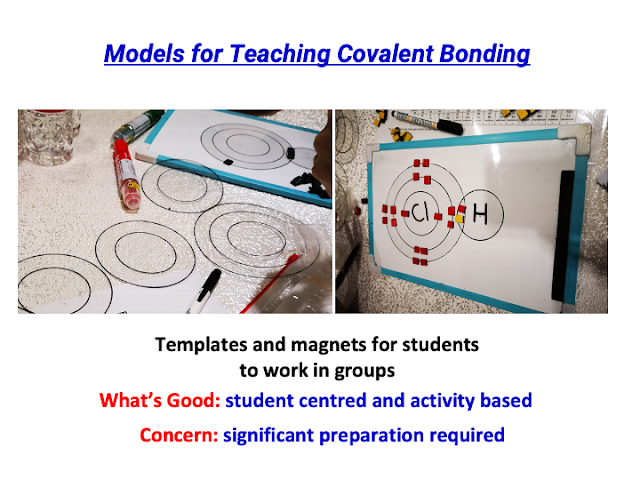
- Did a physical (tactile) version using laminated transparency cards for T&L of Covalent Bonding in visualisation: https://drive.google.com/file/d/1yDFKTuRrquUSNQEGXAYbVHKB1m_3wODD/view?usp=sharing
- so far so good! :)
- Is it possible to have questions for students to complete diagrams for atoms so that they serve to scaffold learning for weaker students or lower sec students?
- Converted to quiz with marks
- Not at the moment (:
- thank you so much.
- Include portion where student need to deduce the number of valence electron
- Thank u so much for the sharing!
- Perhaps can just give proton number instead of valence electron so can make them find out no of valence electrons
- Sometime the electrons are difficult to drag, as in it is not very sensitive. For example, I wanted to drag a blue electron but I keep dragging the green electron that is below it instead
- -
- i hope it can be used on a handphone easily :)
- Can this be embedded in sls
- The atoms have already been pre-arranged for the students. I thought it would be good for them to arrange on their own for some of the molecules like H2O2
- it is a little laggy. Also, possible to be HTML5? Won't use this simulation as at the 'A' levels, we don't draw circles for dot-cross diagrams. Will be good if there could be an option to click to turn on /off the circles. Thanks.
- will perhaps be good if the correct answer will be accepted if pairing of electrons are done accordingly instead of being in a random arrangement
- To include ionic bonding, marcromolecules to cover for pure chemistry students as well.
- Thank you !
- Thanks for the great simulation!
- What about drawing the inner electrons too? So students will be able to understand there's not just valence electrons but also the concept of fully filled inner electron shells.
- Include ionic bonding
- Allows students to explore to discover their own learning.
- Great simulation
- Good that u r working on ionic bond.
- If there is a backend CMS that captures students’ common mistakes , scores etc , would be great
- Na
- For polyatomic ions, is there any way to explain to student?
- Good if the electrons can be free to move
- 1) Really hope that creator can create the simulation for students to show all electrons for ionic bonding / covalent bonding instead of just valence electrons. 2) Suggestion for students to drag and drop electron shells / brackets instead of providing it in the simulation as it give students hints the substances are bonded in certain manner. 3) Providing information such as periodic table / element only for students to decide on the electronic configurations / valence electrons. 4) The dot-and-cross simulation wont be able to provide the 3D arrangement for students to visualize how some of these molecules looks like.
- It will be good if the simulation does not have the circle already drawn. Coz sometimes students have problem knowing how the electrons are shared. E.g. carbonate ion, might not know carbon is in the middle. Or hydrogen peroxide where oxygen should be between the 2 hydrogen.