About
This will be a EJSS-based particle physics simulation
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Software Requirements
| Android | iOS | Windows | MacOS | |
| with best with | Chrome | Chrome | Chrome | Chrome |
| support full-screen? | Yes. Chrome/Opera No. Firefox/ Samsung Internet | Not yet | Yes | Yes |
| cannot work on | some mobile browser that don't understand JavaScript such as..... | cannot work on Internet Explorer 9 and below |
Credits

rytan451; lookang
Sample Learning Goals
Overview
|
Topic: |
Rate of Reaction (Factors affecting Rate of Reaction) |
|
Big idea/ Concept |
|
|
Profile/ Level |
|
|
Prerequisite Knowledge |
|
|
Learning Issue to be addressed |
|
For Teachers

Configure starting and boundary conditions
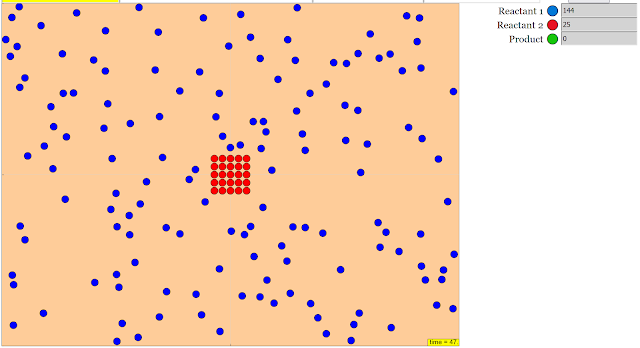
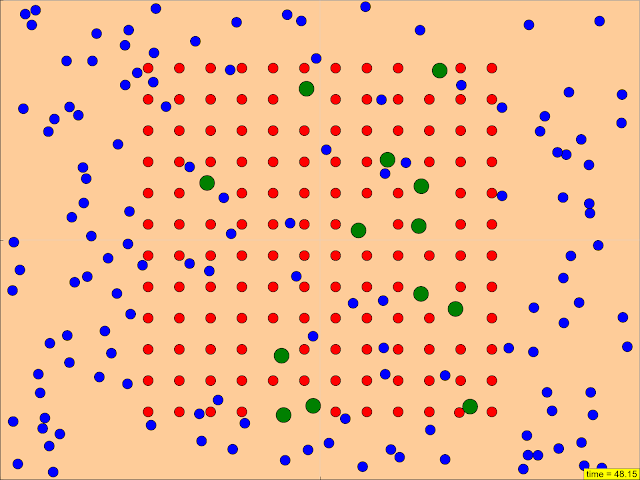
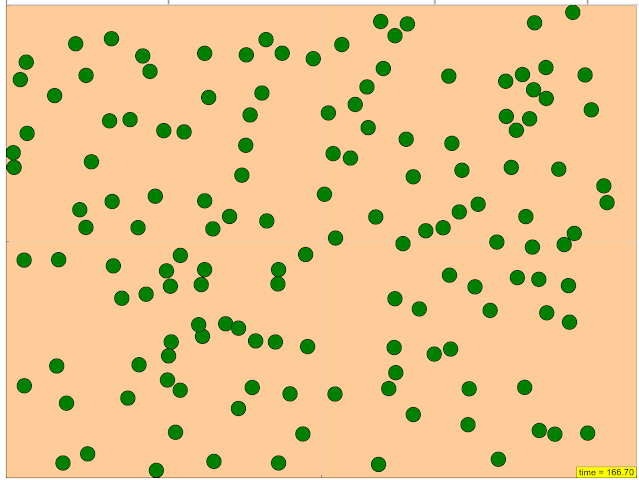
Research
[text]
Video
https://www.youtube.com/watch?v=oeM6hKm6Td4&t=2s Effect of Temperature on Speed of Reaction by ETDtogo
https://www.youtube.com/watch?v=jDmxbFYvQgo How does concentration affect rate of reaction? by ChemJungle
https://www.youtube.com/watch?v=kGJsDDjwP1Y How do Temperature and Concentration Affect the Rate of Reaction? by Chemistry Breakdown
https://www.youtube.com/watch?v=BWN8xVuzuFI The effect of surface area and particle size on the rate of a chemical reaction. by Michael Kavanagh
https://www.youtube.com/watch?v=lukSSS9Hfaw How does surface area affect rate of reaction? by ChemJungle
Version:
Other Resources
https://interactives.ck12.org/simulations/embed.html?embeded=true&interactive=states-of-matter&subject=chemistry&lang=en&assignment=true show the arrangement and movement of particles in the different states of matter are due to the varying strength of the forces of attraction between the particles.
https://interactives.ck12.org/simulations/embed.html?embeded=true&interactive=phases-of-matter&subject=chemistry&lang=en&assignment=true show how solid melts to become a liquid in terms of kinetic particle theory and energy changes!
https://phet.colorado.edu/sims/html/gases-intro/latest/gases-intro_en.html Instructions:
Step 1: Select "Intro"
Step 2: Tick "Collision Counter" tab
Step 3: Expand "Particles" tab by clicking on the "+" icon
Step 4: Add some "Light" (red coloured) particles to the vessel
Step 5: Add in more "Light" particles and observe what happens to:
a. Pressure reading for the vessel
b. Number of Collisions
(remember to start the wall collision counter after you have added the particles and wait for the reading to be taken)
Step 6: Based on your observations from Step 5, go to the "Connect" column below and key in how pressure affects the rate of reaction.
end faq
{accordionfaq faqid=accordion4 faqclass="lightnessfaq defaulticon headerbackground headerborder contentbackground contentborder round5"}
- Details
- Written by Loo Kang Wee
- Parent Category: 03 Chemistry of Reactions
- Category: 03 Chemical Reactions
- Hits: 5657


.png
)






