Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Software Requirements
| Android | iOS | Windows | MacOS | |
| with best with | Chrome | Chrome | Chrome | Chrome |
| support full-screen? | Yes. Chrome/Opera No. Firefox/ Samsung Internet | Not yet | Yes | Yes |
| cannot work on | some mobile browser that don't understand JavaScript such as..... | cannot work on Internet Explorer 9 and below |
Credits



 Written by Loo Kang Wee; Felix J. Garcia Clemente; Francisco Esquembre; Designed by David Loh
Written by Loo Kang Wee; Felix J. Garcia Clemente; Francisco Esquembre; Designed by David Loh
Sample Learning Goals
This simulation is the same as the O level one except for the last question "sulfate(IV) ions, SO₃²⁻"
- A Level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5
- O level Chemical Bonding Dot and Cross Diagrams JavaScript Simulation Applet HTML5
For Teachers
From Google slides (From David) to interactive https://docs.google.com/presentation/d/1fwutLc-jPc1fUyrxJsps3Fhg6_J9y8AEA4pHJ68gdBw/edit?ts=5dd2086a#slide=id.g5292a6c619_0_96
Chemical Bonding Dot and Cross Diagrams
Polyatomic ions dot and cross diagram
|
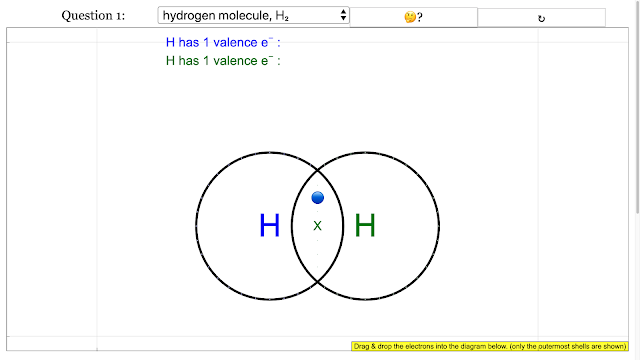
Chemical Bonding Dot and Cross Diagrams for Hydrogen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen molecule answer is add up to 2 on each H atom electron outermost with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Chlorine molecule |
|
Chemical Bonding Dot and Cross Diagrams for Chlorine molecule answer is add up to 8 on each atom's' electron outermost shell with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Oxygen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Oxygen molecule answer is is add up to 8 on each atom's' electron outermost shell with shared electrons = 4 |
|
Chemical Bonding Dot and Cross Diagrams for Nitrogen molecule |
|
Chemical Bonding Dot and Cross Diagrams for Nitrogen molecule answer is is add up to 8 on each atom's' electron outermost shell with shared electrons = 6 |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Chloride molecule |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Chloride molecule answer is is add up to 8 on Cl atom and 2 for H atom and electron outermost shell with shared electrons = 2 |
|
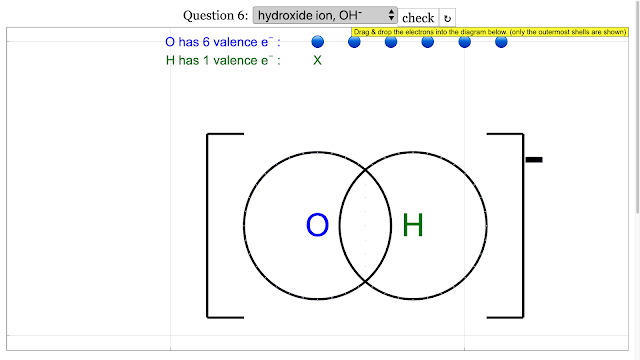
Chemical Bonding Dot and Cross Diagrams for Hydroxide ion |
|
Chemical Bonding Dot and Cross Diagrams for Hydroxide ion answer is is add up to 8 on O atom and 2 for H atom and electron outermost shell with shared electrons = 2 |
|
Chemical Bonding Dot and Cross Diagrams for Cyanide ion |
|
Chemical Bonding Dot and Cross Diagrams for Cyanide ion answer is is add up to 8 on each atoms and electron outermost shell with shared electrons = 6 |
|
Chemical Bonding Dot and Cross Diagrams for Water molecule |
|
Chemical Bonding Dot and Cross Diagrams for Water molecule answer is is add up to 8 on O atom and 2 on H atoms and electron outermost shell with shared electrons = 2,2 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Carbon Dioxide molecule |
|
Chemical Bonding Dot and Cross Diagrams for Carbon Dioxide molecule answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 4,4 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 2,4 respectively with the foreign electron on the O atom with the shared electrons=2 |
|
Chemical Bonding Dot and Cross Diagrams for Nitrite ion answer is is add up to 8 on each atom and electron outermost shell with shared electrons = 2,4 respectively with the foreign electron on the O atom with the shared electrons=2 |
|
Chemical Bonding Dot and Cross Diagrams for Ammonia molecule |
|
Chemical Bonding Dot and Cross Diagrams for Ammonia molecule answer is is add up to 8 on N atom and 2 on H atom and the electron outermost shell with shared electrons = 2,2,2 respectively |
|
Chemical Bonding Dot and Cross Diagrams for Methane molecule |
|
Chemical Bonding Dot and Cross Diagrams for Methane molecule answer is is add up to 8 on C atom and 2 on H atom and the electron outermost shell with shared electrons = 2,2,2, respectively |
|
Chemical Bonding Dot and Cross Diagrams for Carbonate ion |
|
Chemical Bonding Dot and Cross Diagrams for Carbonate ion answer is is add up to 8 on each atom and the electron outermost shell with shared electrons = 4,2,2, respectively. The two O atoms with 2 shared electrons has a foreign electron in it's personal shell. |
 |
|
Chemical Bonding Dot and Cross Diagrams for Hydrogen Peroxide |
Video
[text]
Version:
- https://weelookang.blogspot.com/2019/12/chemical-bonding-dot-and-cross-diagrams.html
- https://sites.google.com/a/mgs.sch.edu.sg/about-me-david-loh/
Other Resources
[text]
end faq
{accordionfaq faqid=accordion4 faqclass="lightnessfaq defaulticon headerbackground headerborder contentbackground contentborder round5"}
- Details
- Written by David Loh
- Parent Category: Chemistry
- Category: 03 Chemistry of Reactions
- Hits: 4262


.png
)