About
Mass spectrometry
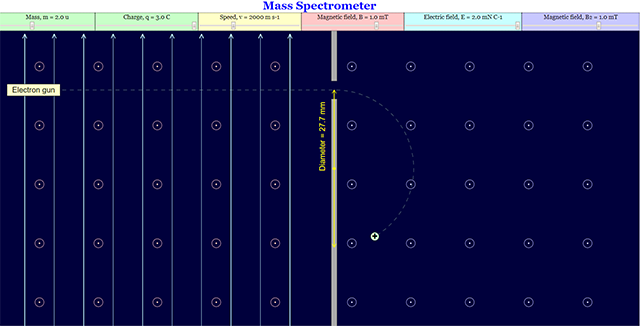
This is the simulation of a mass spectrometer where students are allowed to manipulates mass, charge, speed, magnetic field, electric field
Translations
| Code | Language | Translator | Run | |
|---|---|---|---|---|
 |
||||
Software Requirements
| Android | iOS | Windows | MacOS | |
| with best with | Chrome | Chrome | Chrome | Chrome |
| support full-screen? | Yes. Chrome/Opera No. Firefox/ Samsung Internet | Not yet | Yes | Yes |
| cannot work on | some mobile browser that don't understand JavaScript such as..... | cannot work on Internet Explorer 9 and below |
Credits
 Leong Tze Kwang
Leong Tze Kwang
Sample Learning Goals
Topic/Sub-Topics
PRE-U PHYSICS (H2) (2016) (2016) - 1 selected
-
20(a) infer from the results of the Rutherford α-particle scattering experiment the existence and small size of the atomic nucleus
For Teachers
Initial Page setup. Users are able to change the variables on the top panel.
User can change the mass and the level of the positive charge.
User can change the mass and the charge to a negative charge.
User may also change the speed of the electron.
User can change the view of the Magnetic Field on both sides.
User can enable the view of the Electric Field on the left side.
SLS Lesson History of the atomic model by Leong T.K.
-
Do you know that we are made up of atoms? Do you know we are made up of cells? Do you know that we are on a planet orbiting a sun that is part of a galaxy? Or maybe the world is supported on the backs of giant elephants that stand on the shell of an enormous turtle?In science, unlike in polite conversation, we are quite accustomed to questioning assumptions and the question "Why?" can usually be translated as "How do you know?" [Do be warned that in typical social settings, many people do not take well to having their assumptions directly questioned, so tread carefully.]Up to the end of the 19th Century, we knew that matter was made up of atoms (seemingly indivisible lumps) but no one was able to show that these atoms had internal structure and inner parts.Of course, so much has changed since then -- visible technologies like computers and robots powered fundamentally by our sophisticated understanding of science and mathematics. Not to forget two World Wars, several pandemics including COVID-19, a sobering global stockpile of weapons of mass destruction... but it is always up to us to shape the future.
Dalton's indivisible spheres
When we look around us, everything seems so different. Burning wood turns into fire and ash. To think that oxygen is actually reacting with carbon!John Dalton's breakthrough was realising that so many of the complex chemical processes were fundamentally based on rearrangements of unchanging pieces.Unlike early philosophers like Democritus who imagined and argued for indivisible particle through the impossibility of splitting a chunk of matter indefinitely, Dalton was able to gather robust experimental evidence to support his claims. He focused on experiments with gases and discovered the law of partial pressures: that the combined pressure of a mixture of gases was given by the sum of the partial pressures that each individual gas exerted while occupying the same space.By carefully measuring the relative mass of gases like water vapour, ammonia, and carbon dioxide, in 1803, Dalton’s proposed this atomic model:- Gases are made up of small particles, which are referred to as atoms.
- All atoms of a given element are identical.
- The atoms of different elements vary by mass and other properties.
Building on this work, he and other scientists refined and extended the model, leading to a chart and organisation of atoms, molecules, elements, compounds, and so on. The evidence from further experiments suggested that atoms are indestructible, with chemical reactions resulting in their rearrangement, but not their creation or destruction.Thomson's discovery of electrons
Thomson's discovery of electrons and the plum-pudding model
Until 1897, atoms were believed to be the smallest units of matter.When experimenting with the phenomenon of "cathode rays", J. J. Thomson discovered that these rays were made of charged particles (we call them electrons) because they could be deflected by electric and magnetic fields.He proposed that these cathode rays were ejected from atoms, and that atoms are normally electrically neutral with equal amounts of positive and negative charge. When the atoms eject electrons, they become charged ions.Watch the video below to find out more about Thomson's discovery of electrons, and the delicious-sounding plum-pudding model of the atom.Thomson's Plum Pudding Model of the Atom by Veritasium -
Deflection of an electron beam by a magnetic field
J. J. Thomson used a cathode ray tube similar to what is shown in the video below. He observed that the beam was deflected by both magnetic and electric fields. This allowed him to measure the mass-to-charge ratio of the particles in the beam, which did not depend on the atoms producing the beams.Watch the video and admire the beauty of electron beams. You may wish to use Fleming's left-hand rule (or the right-hand grip convention for the Lorentz force) to analyse the magnetic deflection of the electron for yourself. [Click here if necessary to revise Force on a Moving Charge in a Magnetic Field.] -
-
Rutherford's discovery of the atomic nucleus
Rutherford's discovery
In 1909, Ernest Rutherford, a former student of J. J. Thomson, realised that there was something very dense buried deep within the plum-pudding, which meant that it was not so appropriate to think of it as a plum-pudding anymore. Rather, it seemed like the atom was a microcosm of the solar system!The experiment goes like this...- Prepare some thin gold foil.
- Bombard helium-4 ions (also known as alpha particles) at the foil.
- Most of the alpha particles pass through with small deflections, totally expected, like bullets going through paper.
- But some of these particles bounce right back!
Rutherford deduced that the atoms must have their mass concentrated in a tiny volume (the "nucleus"), such that most alpha particles do not pass very near these concentrations, but some alpha particles happen to pass very near and this basically results in an elastic collision between a relatively low-mass alpha particle and a relatively high-mass gold nucleus!In other words, in this model of the atom, the nucleus was like our Sun and the electrons (discovered by Thomson) were like orbiting planets. Of course, the attractive force was not gravitational but of electromagnetic origin.Watch the video below to find out more about the observations that led to Rutherford's discovery of the atomic nucleus and the planetary model for the atom.Cathode Rays Lead to Thomson's Model of the Atom by VeritasiumAlpha particle scattering by gold nucleus
In the simulation below, you can fire alpha particles and observe their deflection by the gold foil.Click on "Rutherford Atom" to visualise what Rutherford actually observed, and click on "Plum Pudding Atom" to visualise what Rutherford was expecting to observe.There is a button that you need to press to "turn on" the alpha source. You may wish to click the "Traces" checkbox to visualise the trails of the alpha particles. -
Translations
Code Language Translator Run 
Software Requirements
SoftwareRequirements
Android iOS Windows MacOS with best with Chrome Chrome Chrome Chrome support full-screen? Yes. Chrome/Opera No. Firefox/ Samsung Internet Not yet Yes Yes cannot work on some mobile browser that don't understand JavaScript such as.....
cannot work on Internet Explorer 9 and below
Credits



 This email address is being protected from spambots. You need JavaScript enabled to view it.; Anne Cox; Wolfgang Christian; Francisco Esquembre
This email address is being protected from spambots. You need JavaScript enabled to view it.; Anne Cox; Wolfgang Christian; Francisco Esquembre
Video
[text]
Version:
Other Resources
https://vle.learning.moe.edu.sg/moe-library/lesson/view/b6b61e5a-1523-4889-92af-a3b73e21509a?fromCcpm=false
end faq
{accordionfaq faqid=accordion4 faqclass="lightnessfaq defaulticon headerbackground headerborder contentbackground contentborder round5"}
- Details
- Written by Siti
- Parent Category: 02 Static Electricity
- Category: 01 Electromagnetism
- Hits: 6786








.png
)