About
https://phet.colorado.edu/en/simulation/rutherford-scattering
http://weelookang.blogspot.sg/2015/12/rutherford-scattering-by-phet.html
Topics
- Quantum Mechanics
- Atomic Nuclei
- Atomic Structure
- Atoms
- Alpha Particles
- Electric Charges
- Electric Force
- Rutherford Scattering
- Plum Pudding Model
Description
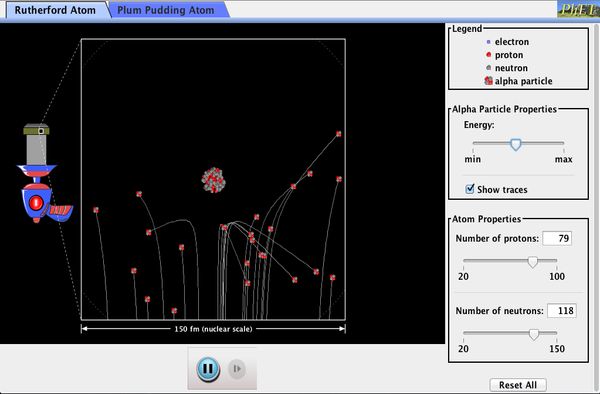
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core.
Sample Learning Goals
- Describe the qualitative difference between scattering off positively charged nucleus and electrically neutral plum pudding atom.
- For charged nucleus, describe qualitatively how angle of deflection depends on: Energy of incoming particle, Impact parameter, Charge of target
For Teachers
[SIMU_TEACHER]
Video
Rutherford's Experiment: Nuclear Atom by uploaded by HerrPingui
Rutherford experiment animation by owigger
Rutherford Gold Foil Experiment - Backstage Science
Software Requirements
| Windows | Macintosh | Linux |
|---|---|---|
|
Microsoft Windows
XP/Vista/7
Sun Java 1.5.0_15 or later
|
OS 10.5 or later
Sun Java 1.5.0_19 or later
|
Sun Java 1.5.0_15 or later |
Credits
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|
end faq
{accordionfaq faqid=accordion3 faqclass="lightnessfaq defaulticon headerbackground headerborder contentbackground contentborder round5"}